Table of Contents
JEE Main Syllabus consists of three papers, including Physics, Chemistry, and Mathematics. The National Testing Agency released the JEE Mains syllabus PDF on its official website. A few topics were removed for students' ease in the JEE Main exam last year itself.
The JEE Main 2025 syllabus consists of important topics such as mathematical reasoning, communication systems, states of matter, s-block elements, and more. The examination questions in the JEE Main exam will be picked from topics covered in the NCERT textbooks of classes 11 and 12.
Thus, candidates must check the JEE Main syllabus PDF download link for different subjects on this page. There will be 90 questions for 300 marks, and the exam will last 3 hours.
JEE Main Syllabus PDF Download
To prepare for the IIT JEE Mains syllabus 2025, applicants must use the NCERT 12th solutions and NCERT 11th solutions. Nonetheless, the entire NTA JEE Main syllabus 2025 link for PDF for Paper I (B.E./B.Tech) and Paper II (B.Arch/B.Plan) is shared below:
| JEE Main Paper | Direct Link |
|---|---|
| JEE Main Paper 1 | Download Here |
| JEE Main Paper 2A | Download Here |
| JEE Main Paper 2B | Download Here |
Read More:
JEE Main Syllabus: Subject-wise Download Link
Candidates can check for the JEE syllabus 2025 pdf links shared below for different courses. The links provided will help them learn the syllabus pattern in detail.
| JEE Main 2025 Paper Wise Syllabus Download | |
|---|---|
| JEE Mains Physics Syllabus 2025 | JEE Main Mathematics Syllabus 2025 |
| JEE Main B.Arch/ B.Planning Syllabus | JEE Main Chemistry Syllabus 2025 |
JEE Mains Syllabus Paper-I (B.E./B.Tech)
A compilation of the JEE Main January 2025 paper 1 syllabus has been mentioned below. To understand the importance of each topic, applicants must check the weightage of chapters in JEE Main.
The JEE Main syllabus 2025 is mapped as per the NCERT textbooks of classes 11 and 12 to ensure uniformity across the country.
JEE Main Syllabus: Physics
Physics is one of the majors of the JEE syllabus 2025. A total of 30 questions will be asked from the entire JEE Main Physics syllabus. There are two sections in JEE Main syllabus 2025 for the Physics paper, as mentioned below:
- Section A contains MCQs and will include the theory with a weightage of 80%.
- Section B contains Numerical Value Questions comprising practical components with a weightage of 20% marks.
Important Link: JEE Main Passing Marks 2025
JEE Main Section A (Theory) Topics
The detailed JEE Mains Physics syllabus 2025 for section A is provided below:
- Thermodynamics
- Physics and measurement
- Rotational motion
- Kinematics
- Work, energy, and power
- Gravel's Law
- Laws of Motion
- Properties of solid
- Oscillations and waves
- Kinetic theory of gases
- Electronic department
- Communication systems
- Magnetic effects of current and magnetism
- Current electricity
- Electromagnetic waves
- Optics
- Electromagnetic induction and alternating currents
- Electrostatics
- Atoms and nuclei
- Dual nature of matter and radiation
Must Practice: JEE Main Previous Year Question Paper
JEE Main Section B (Experimental Skills) Topics
The detailed syllabus topics for the section B (experimental skills) paper of JEE Main 2025 are shared below for applicants' reference.
- Vernier callipers are used to measure the internal and external diameter and depth of a vessel.
- A young module of elasticity of the material of the electric wire.
- Screw gauge-its use to determine the thickness/ diameter of a thin sheet/wire.
- Simple Pendulum-dissipation of energy by plotting a graph between the square of amplitude and time.
- Metre Scale - the mass of a given object by the principle of moments.
- The surface tension of water by capillary rise and the effect of detergents.
- Co-efficient of Viscosity of a given viscous liquid by measuring the terminal velocity of a given sphere.
- Plotting a cooling curve for the relationship between the temperature of a hot body and the time.
- Speed of sound in air at room temperature using a resonance tube.
- Specific heat capacity of a given (i) solid and (ii) liquid by method of mixtures.
- The resistivity of the material of a given wire using a meter bridge.
- Wire using Ohm's law.
- Potential -(i) Comparison of emf of two primary cells.(ii) Determination of internal resistance of a cell.
- Resistance and figure of merit of a galvanometer by half deflection method.
- The focal length of; (i) Convex mirror (ii) Concave mirror, and (ii) Convex lens
- Reflective index of a glass using a traveller.
- The plot of the angle of deviation vs angle of incidence for a triangular prism.
- Characteristic curves of a p-n junction diode in forward and reverse psychology.
- Characteristic curves of a transistor and finding current gain and voltage gain.
- Characteristic curves of a Zener diode and finding reverse breakdown voltage.
- Identification of Diode. LED, Transistor. IC. Resistor. A capacitor from a mixed collection of such items.
- Using a multimeter to:
- Identify the base of a transistor.
- Distinguish between NPN and PNP-type transistors.
- See the unidirectional current in the case of a diode and an LED.
- Check the correctness or otherwise of a given electronic component (diode, transistor, or IC).
JEE Main Physics Important Topics
Candidates can check on the important topic names shared below for Physics for the JEE Mains exam for the year 2025.
| JEE Main Physics Important Topics | |
| Electrostatics | Communication Systems |
| Current Electricity | Semiconductors |
| Capacitors | Electromagnetic Waves |
| Magnetic Effect of Current and Magnetism | Circular Motion |
| Alternating Current | Error in Measurement |
| Kinetic Theory of Gases and Thermodynamics | Wave Optics |
| Simple Harmonic Motion | Elasticity |
| Sound Waves | Modern Physics |
| Kinematics | Rotational Dynamics |
| Work, Energy, and Power | Centre of Mass |
| Laws of Motion | - |
JEE Main Physics Weightage
JEE Mains syllabus 2025 NTA physics topic weightage in the context of the number of questions asked and marks given are shared below.
| Topics | No. of Questions | Total Marks |
| Modern Physics | 5 | 20 |
| Heat and Thermodynamics | 3 | 12 |
| Optics | 3 | 12 |
| Current Electricity | 3 | 12 |
| Electrostatics | 3 | 12 |
| Magnetics | 2 | 8 |
| Unit, Dimension, and Vector | 1 | 4 |
| Kinematics | 1 | 4 |
| Law of Motion | 1 | 4 |
| Work, Power, and Energy | 1 | 4 |
| Centre of Mass, Impulse, and Momentum | 1 | 4 |
| Rotation | 1 | 4 |
| Gravitation | 1 | 4 |
| Simple Harmonic Motion | 1 | 4 |
| Waves | 1 | 4 |
| Electromagnetic Induction | 1 | 4 |
Recommended: JEE Main 2025 Last Minute Preparation Tips
JEE Main Syllabus: Chemistry
The JEE Main syllabus 2025 for chemistry comprises numerical and cleaner questions. A total of 30 questions will be asked from the entire JEE Main exam syllabus. JEE Mains Chemistry syllabus consists of three sections, all of which carry equal weight, including the following:
- Physical Chemistry
- Inorganic Chemistry
- Organic Chemistry
JEE Main Physical Chemistry Syllabus Topics
The detailed NTA JEE Mains syllabus 2025 for the physical chemistry paper can be checked below:
- Chemical bonding and molecular structure.
- States of matter.
- Chemical thermodynamics.
- Some basic concepts in chemistry.
- Atomic structure.
- Solutions.
- Equilibrium.
- Chemical kinetics.
- Surface chemistry.
- Redox reactions and electrochemistry.
JEE Main Inorganic Chemistry Syllabus Topics
Candidates must check the detailed topics for the JEE Mains 2025 inorganic chemistry paper shared below as pointers.
- Co-ordination compounds.
- Hydrogen.
- P block elements group 13 to group 18 elements.
- Classification of elements and periodicity in properties.
- d- and f – block elements.
- Block elements (alkali and alkaline earth metals).
- General principles and processes of isolation of metals.
- Environmental chemistry.
JEE Main Organic Chemistry Syllabus Topics
The chemistry JEE Mains syllabus 2025 topics for the organic paper consist of the following topics:
- Chemistry in everyday life.
- Purification and characterization of organic compounds.
- CFC
- Principles related to practical chemistry.
- Organic compounds containing nitrogen.
- Organic compounds containing halogens.
- Bio-Molecules.
- Organic compounds containing oxygen.
- Polymers.
- Some basic principles of organic chemistry.
Also Read: JEE Main Dress Code 2025
JEE Main Chemistry Important Topics
JEE Main chemistry important topics have been shared below for the candidates. They must be aware of the essential topics that they should focus on more for the preparation of the JEE Mains 2025:
| JEE Main Chemistry Important Topics | |
| Electrochemistry | GOC Isomerism |
| Chemical Bonding | Liquid Solutions |
| Coordination Compound | Alkyl Halides |
| Ionic Equilibrium | Aryl Halides |
| Thermodynamics and Thermochemistry | d-Block Elements |
| Aldehydes and Ketones | p-Block Elements |
| Aromatic Hydrocarbons | Salt Analysis |
JEE Main Chemistry Weightage of Topics
JEE Main 2025 chemistry syllabus consists of topics from Physical, Inorganic, and Organic Chemistry. Candidates can check on the table shared below referring to the weightage of each topic.
| Chemistry Sections | The weightage (%) |
| Physical Chemistry | 35.6% |
| Inorganic Chemistry | 29.7% |
| Organic Chemistry | 24.7% |
JEE Main Syllabus Chemistry Overall Weightage
The number of questions asked and marks allotted for the JEE chemistry syllabus 2025 topics have been shared below in a tabular format for the candidates.
| Topics | No. of Questions | Total Marks |
| Periodic Table and Representative Elements | 3 | 12 |
| Transition Elements and Coordination Chemistry | 3 | 12 |
| Thermodynamics and Gaseous State | 2 | 8 |
| Atomic Structure | 2 | 8 |
| Chemical Bonding | 2 | 8 |
| Chemical and Ionic Equilibrium | 2 | 8 |
| Solid State and Surface Chemistry | 2 | 8 |
| Nuclear Chemistry and Environment | 2 | 8 |
| Mole Concept | 1 | 4 |
| Redox Reaction | 1 | 4 |
| Electrochemistry | 1 | 4 |
| Chemical Kinetics | 1 | 4 |
| Solutions and Colligative Properties | 1 | 4 |
| General Organic Chemistry | 1 | 4 |
| Stereochemistry | 1 | 4 |
| Hydrocarbon | 1 | 4 |
| Alkyl Halides | 1 | 4 |
| Carboxylic Acid and their Derivatives | 1 | 4 |
| Carbohydrates, Amino Acids and Polymers | 1 | 4 |
| Aromatic Compounds | 1 | 4 |
JEE Main Preparation Links:
JEE Main Syllabus: Mathematics
Concept strength, numerical problem-solving skills, and familiarity with number functions go a long way with the JEE Main 2025 mathematics syllabus. The mathematics paper will carry 30 questions in the exam. There will be two sections:
- Section A will comprise Multiple-Choice Questions (MCQs).
- Section B will contain questions whose answers are to be filled in as a numerical value. In Section B, candidates must attempt any five questions out of 10.
The detailed NTA JEE syllabus 2025 for the mathematics paper can be checked below:
| Topics | |
| Complex numbers and quadratic equations | Coordinate geometry |
| Mathematical induction | Trigonometry |
| Sets, relations, and functions | Sequence and Series |
| Limit, continuity, and differentiability | Binomial theorem and its simple applications |
| Matrices and determinants | Statistics and probability |
| Permutations and combinations | Differential equations |
| Integral calculus | Vector Algebra |
| Mathematical reasoning | Three-dimensional geometry |
JEE Main Mathematics Important Topics
Candidates must check on the details shared regarding the important topics of Mathematics essential from the exam point of view.
| JEE Main Maths Important Topics | |
| 3D Geometry | Application of Derivatives |
| Vectors | Conic Sections |
| Determinants and Matrices | Complex Numbers |
| Sequence and Series | Quadratic Equation |
| Circle | Binomial Theorem; |
| Probability | Permutation and Combination |
| Functions | Properties of Triangles |
| Trignometric Equation | - |
NOTE: The candidates should also refer to the previous year's JEE Main question papers to check which topics are important and occupy the maximum weightage.
JEE Main Mathematics Weightage of Topics
JEE Mains syllabus 2025 Mathematics topic-wise weightage has been shared below. The table depicts the number of questions and marks each topic carries in the JEE Main examination:
| Topics | No. of Questions | Total Marks |
| Coordinate Geometry | 5 | 20 |
| Integral Calculus | 3 | 12 |
| Limits, Continuity, and Differentiability | 3 | 12 |
| Vector Algebra | 2 | 8 |
| Statistics and Probability | 2 | 8 |
| Matrices and Determinants | 2 | 8 |
| Quadratic equations | 2 | 8 |
| Complex Numbers | 2 | 8 |
| Three Dimensional Geometry | 2 | 8 |
| Binomial Theorem and its Application | 1 | 4 |
| Sequences and Series | 1 | 4 |
| Definite Calculus | 1 | 4 |
| Differential Equation | 1 | 4 |
| Mathematical Reasoning | 1 | 4 |
| Permutation Combination | 1 | 4 |
| Statistics and Dynamics | 1 | 4 |
| Trigonometry | 1 | 4 |
| Sets, Relations, and Functions | 1 | 4 |
Quick Links:
JEE Main Syllabus for Paper II: B.Arch and B.Plan
JEE Main January 2025 paper 2 syllabus consists of B.Arch and B.Plan courses. Candidates can check on the detailed syllabus for the courses mentioned below:
JEE Main 2025 B.Arch Syllabus for Paper II (A)
JEE Main B.Arch syllabus 2025 consists of topics from Maths, Aptitude, and Drawing. JEE Main Paper II Maths syllabus is the same as the Paper I Maths syllabus. Candidates can check the JEE Main syllabus for aptitude and drawing below:
JEE Main Aptitude Part-I Topics
The JEE Main syllabus 2025 topics for the Aptitude part I of paper II (A) have been shared below for the applicants:
- Awareness of persons, places, Buildings, and Materials
- Objects, Textures related to Architecture, and build-environment
- Visualizing three-dimensional objects from two-dimensional drawings
- Different sides of three-dimensional objects
- Analytical Reasoning Mel Ability (Visual, Numerical, and Verbal)
JEE Main Aptitude Part-II Topics
The JEE Main Aptitude syllabus topics for 2025 are shared below:
- Three-dimensional perception: Understanding and appreciation of scale and proportion of objects, building forms, and elements, colour texture, harmony, and contrast
- Design and drawing of geometrical or abstract shapes and patterns in pencil
- Transformation of forms in both 2-D and 3-D union, subtraction, rotation, development of surfaces and volumes, Generation of plans, elevations and 3-D views of objects
- Creating two-dimensional and three-dimensional compositions using given shapes and forms
JEE Main Drawing Topics
The detailed syllabus topics for the JEE Main drawing paper are mentioned below:
- Sketching of scenes and activities from memory of urbanscape (public space, market, festivals, street scenes, monuments, recreational spaces, etc.), landscape (riverfront, jungles. gardens, trees, plants, etc.), and rural life.
JEE Main Syllabus: B.Plan Paper II (B)
The JEE Main syllabus 2025 for the B.Plan course consists of topics from math, aptitude, and planning. JEE Main complete maths syllabus is the same as the Paper I maths syllabus, and JEE Main's syllabus for aptitude is the same as B.Arch Paper II(A). Candidates can check the JEE Main syllabus for planning below:
JEE Main General Awareness Syllabus Topics
The JEE Main syllabus 2025 topics for the general awareness unit for paper II (B) is listed below for the applicants reference.
- General knowledge questions and knowledge about prominent cities
- Development issues
- Government programs.
JEE Main Preparation Links:
JEE Main Social Science Syllabus
The syllabus topics for JEE Main Paper II (B) social science include national economy, globalization, colonialism, and many more. Applicants must check the detailed list shared below:
- The idea of nationalism
- Nationalism in India
- The pre-modern world
- 19th -century global economy
- Colonialism
- Colonial cities
- Industrialization
- Resources
- Development
- Agriculture, Water, and Mineral Resources
- Industries
- National economy
- Human Settlements
- Power-sharing
- Federalism
- Political Parties
- Democracy
- The constitution of India
- Economic development- economic sectors
- The concept of development
- Population structure
- Poverty
- Social exclusion
- Inequality
JEE Main Thinking Skills Topics
The thinking skills paper of JEE Main 2025 for B. Plan paper II (B) is listed below for the candidates:
- Comprehension (unseen passage)
- Map reading skills
- Scale
- Distance
- Direction
- Area
- Critical reasoning
- Understanding of charts
- Graphs and Tables
- Basic concepts of statistics and quantitative reasoning
Note: All candidates are advised to bring their pencils, geometry box set, erasers, coloured pencils, and crayons for the Aptitude Test inside the examination hall.
Also Read:
Will JEE Main Syllabus be Reduced?
NTA has announced a reduction in JEE Main Syllabus 2025 for the academic year 2025 - 26. Based on the notification, a few topics have been reduced from the NTA JEE Mains syllabus 2025.
Applicants must check the list for the JEE reduced syllabus 2025 details for all the subjects:
JEE Main Syllabus: Mathematics Removed Topics
A few topics are removed from each section of the syllabus of JEE Mains 2025. Candidates can check the JEE Mains reduced syllabus 2025 below for the Mathematics paper:
- Mathematical Inductions
- Mathematical Reasoning
- Few subtopics from Three Dimensional Geometry
A few chapters have been unmerged in the JEE Main syllabus 2025, including Sets, Relations and Functions; these are now two different chapters “Sets and Relations” and “Functions.” Similarly, “Vectors and Three-dimensional Geometry” and “Linear Programming” have been merged into a single chapter.
JEE Main Syllabus: Physics Removed Topics
Applicants must check the list of topics for the reduced syllabus of JEE 2025 for the Physics paper shared below:
- Communication Device
- Capacitors and Capicitance
- Few subtopics from Experimental Skills
“Electromagnetic Induction and Altering Currents” and “Optics” are now a single chapter.
JEE Main Syllabus: Chemistry Removed Topics
The JEE Mains 2025 syllabus reduced topic list for the Chemistry paper has been mentioned below for the applicants:
- Physical quantities and their measurements in Chemistry, Precision and accuracy, significant figures
- States of Matter
- Thomson and Rutherford's Atomic Models and Their Limitation
- Surface Chemistry
- s-Block Elements
- General Principles and Processes of Isolation of Metals
- Hydrogen
- Environmental Chemistry
- Polymers
- Chemistry in Everyday Life
Read More: JEE Main 2025 Reduced Syllabus
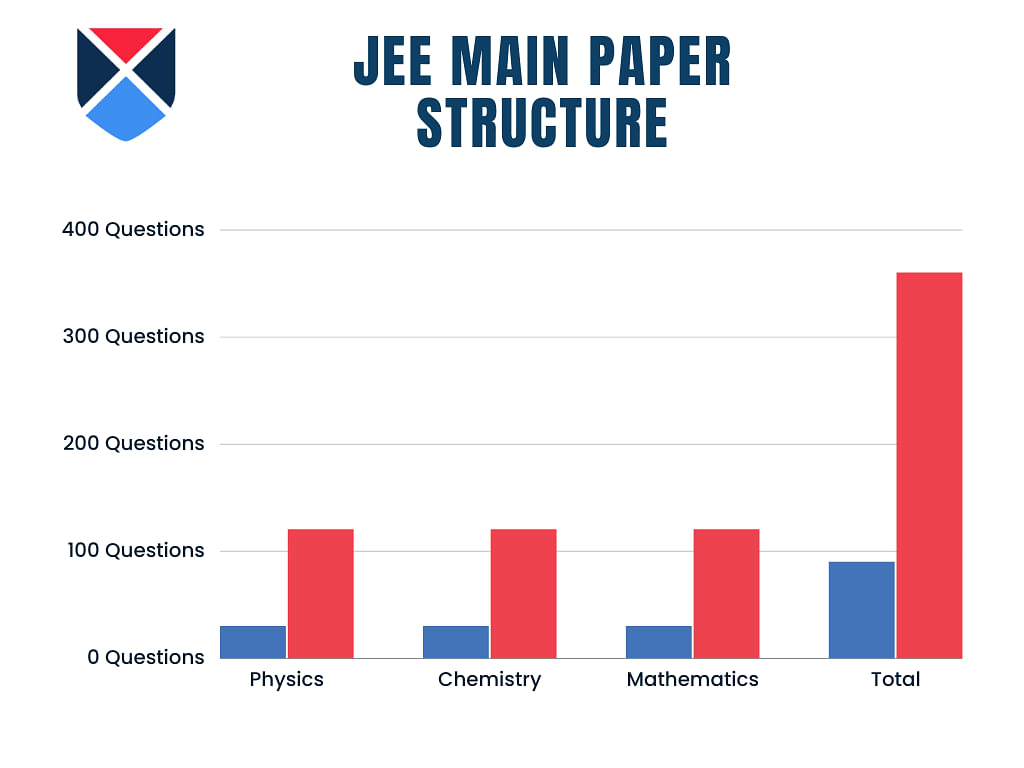
JEE Main Paper Structure
The JEE Main 2025 exam paper structure decided by the National Testing Agency (NTA) is given below. The time duration of JEE Main exam is 3 hours. Candidates are given 90 questions to attempt within the set time, overall containing 360 marks.
|
Subjects |
Total Number of Questions |
Total Marks |
|
Physics |
30 Questions |
120 Marks |
|
Chemistry |
30 Questions |
120 Marks |
|
Mathematics |
30 Questions |
120 Marks |
|
Total |
90 Questions |
360 Marks |
JEE Main Syllabus vs JEE Advanced Syllabus
For those wondering whether the JEE Mains and Advanced syllabus are the same, the differences between the JEE Main and Advanced syllabus are given below.
Candidates must learn that the topics that are not mentioned below in the JEE Mains 2025 syllabus are asked in the JEE Advanced examination.
Difference in Physics Syllabus
NTA JEE 2025 syllabus for Physics is listed below in a tabular format for the candidates but won't be included in the JEE Advanced syllabus 2025.
- Electronic Waves: Characteristics of Electronic Waves, Transverse Nature of Electronic Waves, Application of EM Waves, Electromagnetic Spectrum.
- Electronic Devices: Semiconductors, Reverse Bias and Forward Bias, Characteristics of Photo-Diode (I-V), LEDs and Solar Cells, Zener Diode as a Voltage Regulator, Junior Transistors, Transistor Action, Transistors as Amplifiers and Switches, Oscillators.
- Communication Systems: Propagation of EM Waves, Amplitude and Frequency Modulation, Signal Bandwidth and Transmission Medium, Basic Elements of Communication Systems (Block Diagrams).
Difference in Chemistry Syllabus
Below is a list of the Chemistry syllabus for JEE Mains 2025, and these would not be a part of the JEE Advanced syllabus.
- Biomolecules: Definition and Classification of Biomolecules, Functions of Biomolecules, Nucleic Acids and their Composition, DNA, RNA and functions.
- Chemistry in Everyday Life: Cleansing Action of Soaps and Detergents, Artificial Chemicals in Food, Analgesics, Tranquillizers, Antibiotics, Antiseptics, Antifertility Drugs, Disinfectants, Antimicrobials, Antihistamines
Difference in Mathematics Syllabus
The JEE math syllabus 2025 consists of trigonometry, probability and statistics, etc. The details list for the same has been tabulated below. However, these topics will not be a part of the JEE Advanced syllabus for 2025.
- Trigonometry: Heights and Distances.
- Probability and Statistics: Mean and Median, Measures of Dispersion, Calculating the mode of grouped and ungrouped data, Calculating variance, the mean and standard deviation of grouped or ungrouped data.
- Mathematical Reasoning: Tautology, Converse, Contradiction, Contrapositive, Statements and Logical Operations.
- Sets, Relations and Functions: Representations, Unions, intersections and compliments, Power sets, Algebraic Properties of Sets, Types of relations, and Equivalent relations.
Must Read: Most Easy and Scoring Chapters for JEE Main 2025
JEE Main Previous Year Syllabus PDF Link
JEE Main 2025 syllabus pdf download links are given below for candidates' reference:
| JEE Main Paper | Direct Link |
|---|---|
| JEE Main Paper 1 | Download PDF |
| JEE Main Paper 2A | Download PDF |
| JEE Main Paper 2B | Download PDF |
Candidates can check out the previous year's (2023, 2022, and 2021) syllabus PDF links shared below for reference.
| Particulars | PDF Link |
| JEE Main 2023 Syllabus | Download PDF |
| JEE Main 2022 Syllabus | Download PDF |
| JEE Main 2021 Syllabus | Download PDF |
FAQs on JEE Main Syllabus
Q: What is the JEE Main syllabus 2025?
Q: How to download the JEE Mains 2025 syllabus with weightage PDF?
Q: Will the JEE Main 2025 syllabus and JEE Advanced syllabus be the same?
Q: Is the JEE Mains syllabus 2025 the same as CBSE Class 12?
Q: Can there be a reduction in the JEE Mains 2025 syllabus?