Table of Contents
BITSAT Chemistry Syllabus 2025 includes States of Matter, Atomic Structures, Thermodynamics, Electrochemistry, etc. Students can refer to the official BITSAT website for the BITSAT Syllabus 2025 PDF. A total of 30 questions are asked from Chemistry syllabus in the BITSAT exam.
The Chemistry BITSAT weightage 2025 aids students in strategising for the BITSAT exam 2025. Students must strengthen high-weight topics to cover a significant part of the BITSAT syllabus. Candidates can read the Chemistry BITSAT question 2025 weightage below.
Download: BITSAT Previous Year Question Paper
BITSAT Chemistry Syllabus 2025 PDF
Students may visit the official BITSAT website at bitsadmission.com to view the syllabus. Students must compare the topics to the current knowledge bank to identify weak areas. Students can apply the information from the syllabus and weightage sections to prioritise essential topics.
The BITSAT Chemistry syllabus 2025 is expected to be moderate in difficulty. Students must include the BITSAT mock tests 2025 and revise the topics as they study them.
The official BITSAT website has the syllabus for inorganic and organic chemistry for BITSAT 2025. Students may also review and save the same from the table below. To download the BITSAT 2025 syllabus PDF for Chemistry, click on the link and then the download tab on the top-right side corner of the PDF.
| Download BITSAT Chemistry Syllabus 2025 PDF | Download PDF |
Also Download:
- BITSAT Mathematics Syllabus 2025
- BITSAT Physics Syllabus 2025
- BITSAT English Syllabus 2025
- BITSAT Biology Syllabus 2025
BITSAT 2025 Chemistry Syllabus
The detailed topic-wise BITSAT exam Chemistry syllabus is listed for students. Some important topics include States of Matter, Atomic Structures, Principles of Organic Chemistry, and Hydrocarbons. In case of any changes by BITSAT authorities, this page shall be updated.
States of Matter:
- Measurement: Physical Quantities and Si Units, Dimensional Analysis, Precision, Significant Figures.
- Chemical Reactions: Laws of Chemical Combination, Dalton’s Atomic Theory. Close Packing.
- Crystal Structures: Simple Ab and AB2 Type Ionic Crystals, Covalent Crystals – Diamond & Graphite, Metals. Mole Concept.
- Atomic, Molecular and Molar Masses. Percentage Composition Is an Empirical & Molecular Formula.
- Types of Bonding, Melting and Boiling Points. Gaseous State: Gas Laws, Ideal Behaviour, Ideal Gas Equation, Empirical Derivation of Gas Equation.
- Avogadro Number, Kinetic Theory – Maxwell Distribution of Velocities, Average, Root Mean Square.
- Deviation From Ideal Behaviour. Critical Temperature, Liquefaction of Gases, Van Der Waals Equation.
- Liquid State: Vapour Pressure, Surface Tension, Viscosity. Solid State: Classification.
- Space Lattices & Crystal Systems. Balanced Chemical Equations & Stoichiometry.
- Three States of Matter, Intermolecular Interactions, a Unit Cell in Two-Dimensional and Three-Dimensional Lattices, Calculation of Density of Unit Cell – Cubic & Hexagonal Systems.
- Voids, Several Atoms per Unit Cell in a Cubic Unit Cell, Imperfections, Point Defects, and Non-stoichiometric Crystals.
- Electrical, Magnetic and Dielectric Properties.
- Amorphous Solids – Qualitative Description.
- Band Theory of Metals, Conductors, Semiconductors and Insulators, and N- And P-Type Semiconductors.
Atomic Structure:
- Introduction: Radioactivity, Subatomic particles. Atomic number, isotopes and isobars. Periodicity: Brief history of the development of periodic tables Periodic law & the modern periodic Table.
- Many electron atoms: Pauli exclusion principle.
- Quantum mechanics: Wave-particle duality – de Broglie relation, Uncertainty principle.
- Thompson’s model and its limitations.
- Hydrogen atom spectrum and Bohr model and its limitations.
- Hydrogen atom: Quantum numbers and wavefunctions, atomic orbitals & their shapes (s, p, d), Spin quantum number.
- Aufbau principle & the electronic configuration of atoms, Hund’s rule.
- Periodic trends: ionisation energy, atomic, & ionic radii, inter gas radii, electron affinity, electronegativity & valency.
- Types of elements: s, p, d, & f blocks.
- Nomenclature of elements with atomic number greater than 100.
Chemical Bonding & Molecular Structure:
- Valence Electrons, Ionic Bond: Lattice Energy & Born-Haber Cycle.
- Covalent Character of Ionic Bonds & Polar Character of a Covalent Bond, Bond Parameters.
- Molecular Structure: Lewis Picture & Resonance Structures, Vsepr Model & Molecular Shapes.
- Covalent Bond: Valence Bond Theory- Orbital Overlap, Directionality of Bonds & Hybridization (S, P & D Orbitals Only), Resonance.
- Bond Order, Magnetic Properties for Homonuclear Diatomic Species (Qualitative Idea Only).
- Metallic Bond: Qualitative Description.
- Molecular Orbital Theory- Methodology, Orbital Energy Level Diagram Intermolecular.
- Forces: Polarity. Dipole Moments. Hydrogen Bond.
Thermodynamics:
- Basic Concepts: Systems & Surroundings.
- State Functions. Intensive & Extensive Properties. Xv Zeroth Law & Temperature.
- First Thermondynamic Law: Work, Internal Energy, Heat, Enthalpy, Heat Capacities & Specific Heats.
- Measurements of ∆U & ∆H, Enthalpies of Formation, Phase Transformation, Ionisation, Electron Gain. Thermochemistry.
- Hess’s Law, Enthalpy of Bond Dissociation, Combustion, Atomization, Sublimation, Solution & Dilution.
- Second Law: Spontaneous & Reversible Processes. Entropy.
- Gibbs Free Energy Related to Spontaneity & Non-spontaneity, Non-mechanical Work.
- Standard Free Energies of Formation, Free Energy Change & Chemical Equilibrium.
- Third Law: Introduction.
Physical & Chemical Equilibria:
- Osmotic Pressure, Determination of Molecular Mass.
- Concentration Units: Mole Fraction, Molarity, & Molality Solutions: Solubility of Solids & Gases in Liquids, Vapour Pressure, Raoult’s Law, Relative Lowering of Vapour Pressure, Depression in Freezing Point.
- Elevation in Boiling Point. Solid Solutions, Abnormal Molecular Mass. Hydrolysis. Solubility Product of Sparingly Soluble Salts.
- Hoff Factor. Factors Affecting Equilibria: Concentration, Temperature, Pressure, Catalysts, Significance of G & G0 in Chemical Equilibria.
- Equilibrium: Dynamic Nature, Law of Mass Action.
- Chemical Equilibria: Equilibrium Constants (KP, Kc), Factors Affecting Equilibrium, Lechatelier’s Principle.
- Physical Equilibrium: Equilibria Involving Physical Changes (Solid-Liquid, Liquid-Gas, Solid-Gas).
- Surface Chemistry, Adsorption, Physical & Chemical Adsorption, Langmuir Isotherm, Colloids & Emulsion, Classification, Preparation, Uses.
- Ionic Equilibria: Strong & Weak Electrolytes, Acids & Bases (Arrhenius, Lewis, Lowry & Bronsted) & Their Dissociation.
- Degree of Ionisation, Ionization of Water. Ionisation of Polybasic Acids, PH. Buffer Solutions. Henderson Equation, Acid-Base Titrations. Common Ion Effect.
Electrochemistry:
- EMF of Galvanic Cells. Nernst Equation.
- Factors Affecting the Electrode Potential.electrode Potential & Electrolysis, Commercial Production of the Chemicals, Naoh, NA, Al, CL2 & F2.
- Redox Reactions: Oxidation-Reduction Reactions (Electron Transfer Concept).
- Electrolysis, Faraday’s Laws of Electrolysis. Oxidation Number. Balancing of Redox Reactions.
- Electrochemical Cells & Cell Reactions. Standard Electrode Potentials.
- Gibbs Energy Change & Cell Potential.
- Secondary Cells. Dry Cells, Fuel Cells. Electrolytic Conduction: Electrolytic Conductance.
- Specific & Molar Conductivities. Corrosion & Its Prevention.
- Variations of Conductivity With Concentration, Kolhrausch’s Law & Its Application. Coulometer.
Chemical Kinetics:
- Aspects of Kinetics: Rate & Rate Expression of a Reaction. Rate Constant.
- Factor Affecting the Rate of the Reactions: Concentration of the Reactants, Catalyst.
- Integrated Rate Expressions & Half-Life for Zero & First Order Reactions. Order & Molecularity of the Reaction.
- The Size of Particles, the Temperature Dependence of Rate Constant Concept of Collision Theory (Elementary Idea, No Mathematical Treatment).
- Factors Affecting the Rate of Collisions Between Molecules. Mechanism of Reaction: Elementary Reactions.
- Complex Reactions. Reactions Involving Two/Three Steps Only.
- Activation Energy. Catalysis, Surface Catalysis, Enzymes, Zeolites. Surface Chemistry Adsorption – Physisorption & Chemisorption.
- Factors Affecting Adsorption of Gasses on Solids. Catalysis: Homogenous & Heterogeneous, Activity & Selectivity: Enzyme Catalysis, Colloidal State: A Distinction Between True Solutions, Colloids & Suspensions. Emulsions – Types of Emulsions.
- Lyophilic, Lyophobic Multimolecular & Macromolecular Colloids. Properties of Colloids. Tyndall Effect, Brownian Movement, Electrophoresis, Coagulations.
Hydrogen & s-block elements:
- Hydrogen: Element: Unique Position in Periodic Table, Occurrence, Isotopes. Dihydrogen: Preparation, Properties, Reactions, & Uses.
- Molecular, Saline, Ionic, Covalent, Interstitial Hydrides. Water: Properties. Structure & Aggregation of Water Molecules. Heavy Water.
- S-Block Elements: Abundance & Occurrence. Anomalous Properties of the First Elements in Each Group. Diagonal Relationships. Hydrogen Peroxide: Preparation, Reaction, Structure & Use, Hydrogen as a Fuel.
- Trends in the Variation of Properties (Ionisation Energy, Atomic & Ionic Radii). Biological Importance.
- Reactions With Oxygen, Hydrogen, Halogens Water & Liquid Ammonia.
- Alkali Metals: Lithium, Sodium & Potassium: Occurrence, Extraction, Reactivity, & Electrode Potentials.
- Basic Nature of Oxides & Hydroxides. Halides. Properties & Uses of Compounds Such as NaCl, Na2CO3, NaHCO3, NaOH, KCl, & KOH.
- Alkaline Earth Metals: Magnesium & Calcium: Occurrence, Extraction, Reactivity & Electrode Potentials.
- Reactions with O2, H2O, H2 & Halogens. Solubility & Thermal Stability of oxo salts.
- Biological Importance of Ca & Mg.
- Preparation, Properties & Uses of Important Compounds such as CaO, Ca(OH)2, Plaster of Paris, MgSO4, MgCl2, CaCO3, & CaSO4. Lime & Limestone, Cement.
p-block, d-block & f-block elements:
- General: Abundance, Distribution, Physical & Chemical Properties, Isolation & Uses of Elements. Trends in Chemical Reactivity of Elements of a Group. Electronic Configuration, Oxidation States Anomalous Properties of the First Element of Each Group.
- Group 13 Elements: The Reaction of Aluminium With Acids & Alkalis. Boron. Properties & Uses of Borax, Boric Acid, Boron Hydrides & Halides.
- Group 14 Elements: Silicon: Silica, Silicates, Silicone, Silicon Tetrachloride, Zeolites, & Their Uses. Carbon: Carbon Catenation, Physical & Chemical Properties, Uses, Allotropes (Graphite, Diamond, Fullerenes), Oxides, Halides & Sulphides, Carbides.
- Group 15 Elements: Oxides of Nitrogen & Their Structures. Ammonia: Haber’s Process, Properties & Reactions. Dinitrogen. Preparation, Reactivity & Uses of Nitrogen. Compound of Nitrogen. Industrial & Biological Nitrogen Fixation. Properties & Ostwald’s Process of Nitric Acid Production. Fertilisers – Npk Type. Production of Phosphorus. Allotropes of Phosphorus. Preparation, Structure & Properties of Hydrides, Oxides, Oxoacids (Elementary Idea Only) & Halides of Phosphorus, Phosphine.
- Group 16 Elements: Preparation, Structure & Properties of Ozone. Isolation & Chemical Reactivity of Dioxygen. Acidic, Basic & Amphoteric Oxides. Preparation/Production Properties & Uses of Sulphur Dioxide & Sulphuric Acid. Structure & Properties of Oxides, Oxoacids (Structures Only), Hydrides & Halides of Sulphur. Allotropes of Sulphur.
- Group 17 & Group 18 Elements: Inter Halogen Compounds. Bleaching Powder. Structure & Properties of Hydrides, Oxides, Oxoacids of Halogens (Structures Only). Preparation, Properties & Uses of Chlorine & Hcl. Uses of Group 18 Elements, Preparation, Structure & Reactions of Xenon Fluorides, Oxides, & Oxoacids. D-Block Elements: General Trends in the Chemistry of the First-Row Transition Elements. Metallic Character.
- Oxidation State. Ionisation Enthalpy. Ionic Radii. Color. Catalytic Properties. Magnetic Properties. Interstitial Compounds.
- Occurrence & Extraction of Iron, Copper, Silver, Zinc, & Mercury. Alloy Formation. Steel & Some Important Alloys.
- Preparation & Properties of CUSO4, 2cr2o7, KMNO4, Mercury Halides. Silver Nitrate & Silver Halides. Photography.
- F-Block Elements: Lanthanoids & Actinoids. Oxidation States & Chemical Reactivity of Lanthanoids Compounds. Lanthanide Contraction & Its Consequences, Comparison of Actinoids & Lanthanoids.
- Coordination Compounds: Coordination Number. Ligands. Werner’s Coordination Theory. Iupac Nomenclature.
- Application & Importance of Coordination Compounds (In Qualitative Analysis, Extraction of Metals & Biological Systems e.g. Chlorophyll, Vitamin B12, & Haemoglobin).
- Bonding: Valence-Bond Approach, Crystal Field Theory (Qualitative). Stability Constants. Shapes, Colour & Magnetic Properties. Isomerism Including Stereoisomerism. Organometallic Compounds.
Principles of Organic Chemistry & Hydrocarbons:
- Classification: General Introduction, Classification Based on Functional Groups, Trivial & Iupac Nomenclature.
- Electronic Displacement in a Covalent Bond: Inductive, Resonance Effects, & Hyperconjugation. Free Radicals.
- Methods of Purification: Qualitative & Quantitative. Petroleum: Composition & Refining, Uses of Petrochemicals.
- Carbocations, Carbanions, Nucleophiles & Electrophiles. Types of Organic Reactions, Free Radical Halogenations.
- Alkanes & Cycloalkanes: Structural Isomerism, General Properties & Chemical Reactions, Free Radical Halogenation, Combustion & Pyrolysis.
- Alkenes & Alkynes: General Methods of Preparation & Reactions, Acidic Character of Alkynes & (1,2 & 1,4) Addition to Dienes, Physical Properties, Electrophilic & Free Radical Additions.
- Aromatic Hydrocarbons: Isomerism. Resonance Delocalization. Aromaticity. Sources. Properties.
- Polynuclear Hydrocarbons. Iupac Nomenclature. Mechanism of Electrophilic Substitution Reaction, Directive Influence & Effect of Substituents on Reactivity.
- Carcinogenicity & Toxicity. Haloalkanes & Haloarenes: Physical Properties, Nomenclature, Optical Rotation, Chemical Reactions and Mechanism of Substitution Reaction.
- Uses & Environmental Effects. Di, Tri, Tetrachloromethane, Iodoform, Freon & DDT.
Stereochemistry:
- Introduction: Chiral Molecules. R,S & D,L configurations.newman & Sawhorse Projections.
- Optical Activity. Fischer Projections. Enantiomerism. Polarimetry. Racemates. Diastereomeric & Mesostructures.
- Conformations: Ethane Conformations. Geometrical Isomerism in Alkenes.
Organic Compounds with Functional Groups Containing Oxygen & Nitrogen:
- General: Nomenclature, Electronic Structure, Uses of Alcohols, Phenols, Identification, Important Reactions, Important Methods of Preparation, Physical & Chemical Properties, Carboxylic Acids, Nitro Compounds, Amines, Diazonium Salts, Cyanides & Isocyanides Ethers, Aldehydes, Ketones.
- Specific: Reactivity of -Hydrogen in Carbonyl Compounds, an Effect of Substituents on Alpha-Carbon on Acid Strength, Comparative Reactivity of Acid Derivatives, Importance of Diazonium Salts in Synthetic Organic Chemistry.
- Mechanism of Nucleophilic Addition & Dehydration, the Basic Character of Amines, Methods of Preparation, & Their Separation.
Biological, Industrial & Environmental Chemistry:
- The Cell: Concept of Cell & Energy Cycle. Carbohydrates: Classification.
- Structures of Pentoses & Hexoses. Anomeric Carbon. Mutarotation. Monosaccharides. Simple Chemical Reactions of Glucose, Disaccharides: Reducing & Nonreducing Sugars – Sucrose, Maltose & Lactose.
- Polysaccharides: An Elementary Idea of Structures of Starch, Cellulose & Glycogen.
- Proteins: Amino Acids. Denaturation of Proteins & Enzymes. Peptide Bond.
- Polypeptides. Primary Structure of Proteins. Simple Idea of Secondary, Tertiary & Quarternary Structures of Proteins.
- Nucleic Acids: Types of Nucleic Acids. Primary Building Blocks of Nucleic Acids (Chemical Composition of Dna & Rna). Transcription & Protein Synthesis. Genetic Code. Primary Structure of Dna & Its Double Helix. Replication.
- Vitamins: Classification, Structure, Functions in Biosystems. Hormones Polymers: Classification of Polymers. General Methods of Polymerization. The Molecular Mass of Polymers. Biopolymers & Biodegradable Polymers. Methods of Polymerization (Free Radical, Cationic & Anionic Addition Polymerizations).
- Copolymerization: Natural Rubber. Condensation Polymers. Vulcanization of Rubber. Synthetic Rubbers.
- Pollution: Environmental Pollutants. Soil, Water & Air Pollution. Smog. Major Atmospheric Pollutants. Acid Rain. Chemical Reactions in Atmosphere. Ozone & Its Reactions. Depletion of the Ozone Layer & Its Effects. Industrial Air Pollution. Green Chemistry, Study for Control of Environmental Pollution.
- Chemicals in Medicine, Health-Care & Food: Analgesics, Tranquillizers, Antiseptics, Disinfectants, Greenhouse Effect & Global Warming. Antimicrobials, Antifertility Drugs, Antihistamines, Antibiotics, Antacids. Preservatives, Artificial Sweetening Agents, Antioxidants, Soaps & Detergents.
Theoretical Principles of Experimental Chemistry:
- Volumetric Analysis: Principles. Standard Solutions of Sodium Carbonate & Oxalic Acid. Redox Reactions Involving KI, h2so4, na2so3, na2s2o3 & H2s.
- Potassium Permanganate in Acidic, Basic & Neutral Media. Titrations of Oxalic Acid, Ferrous Ammonium Sulphate With KMnO4, K2 Cr2O7/Na2S2O3, Acid-base Titrations. Cu(II)/Na2S2O3.
- Qualitative Analysis of Inorganic Salts: Principles in the Determination of the Cations Pb2+, Cu2+, As3+, Mn2+, Al3+, Zn2+, Co2+, Ca2+, Sr2+, Ba2+, Mg2+, NH4+, Fe3+, Ni2+ & the anions CO32-, S2-, SO42-, SO32-, NO2-, NO3-, Cl-, Br-, I-, PO43-, CH3COO-, C2O42-.
- Physical Chemistry Experiments: Preparation & Crystallisation of Alum, Copper Sulphate. Benzoic
- Acid Ferrous Sulphate, Double Salt of Alum & Ferrous Sulphate, Potassium Ferric Sulphate. Temperature vs. Solubility. Study of PH Charges by Common Ion Effect in the Case of Weak Acids & Weak Bases.
- PH Measurements of Some Solutions Obtained From Fruit Juices, Solutions of Known & Varied Concentrations of Acids, Bases & Salts Using PH Paper or Universal Indicator. Lyophilic & Lyophobic Sols.
- Dialysis. Role of Emulsifying Agents in Emulsification. Equilibrium Studies Involving Ferric & Thiocyanate Ions (Ii) [Co(H2O)6] 2+ & Chloride Ions. Enthalpy Determination for Strong Acid vs. Strong Base Neutralisation Reaction (Ii) Hydrogen Bonding Interaction Between Acetone & Chloroform. Rates of the Reaction Between (I) Sodium Thiosulphate and Hydrochloric Acid, (II) Potassium Iodate & Sodium Sulphite (Iii) Iodide vs. Hydrogen Peroxide, Concentration & Temperature Effects in These Reactions.
- Purification Methods: Filtration, Crystallisation, Sublimation, Distillation, Differential Extraction, & Chromatography.
- Principles of Melting Point & Boiling Point Determination. Principles of Paper Chromatographic Separation – Rf Values.
- Qualitative Analysis of Organic Compounds: Detection of Nitrogen, Sulphur, Phosphorus & Halogens. Detection of Carbohydrates, Fats & Proteins in Foodstuff. Detection of Alcoholic Phenolic, Aldehydic, Ketonic, Carboxylic, Amino Groups & Unsaturation.
- Quantitative Analysis of Organic Compounds: Basic Principles for the Quantitative Estimation of Carbon, Hydrogen, Nitrogen, Halogen, Sulphur & Phosphorous. Molecular Mass Determination by Silver Salt & Chloroplatinate Salt Methods. Calculations of Empirical & Molecular Formulae.
- Principles of Organic Chemistry Experiments: Preparation of Iodoform, Acetanilide, P-Nitro Acetanilide, Di-Benzyl Acetone, Aniline Yellow, Beta-Naphthol.
- Preparation of Acetylene & Study Of Its Acidic Character. Basic Laboratory Technique: Cutting Glass Tube & a Glass Rod, Bending a Glass Tube, Drawing Out a Glass Jet, Boring of Cork.
Also Read:
Chemistry BITSAT Weightage 2025
The table below shows the chemistry weightage for BITSAT 2025. The Chemistry weightage in BITSAT 2025 signifies which topics are most important in the exam. The higher the number of questions, the higher the weightage of the subjects.
Thus, students must concentrate on high-weightage subtopics to cover the crucial portion of the BITSAT Chemistry Syllabus 2025. The BITSAT chemistry chapter wise weightage is tabulated below:
| Chemistry Topics | Question Weightage |
| p-block Element | 4-5 |
| Equilibria | 3-4 |
| Basic Concepts | 5-6 |
| Aldehydes, Ketones & Carboxylic Acids | 3-4 |
| Chemical Bonding | 3-4 |
| Coordination Compounds | 2-3 |
| The Solid State | 2-3 |
| Alcohols, Phenols & Ethers | 3-4 |
| Phenols | 2-3 |
| Chemical Kinetics | 2-3 |
| Hydrogen & s-block Element | 2-3 |
| Polymers | 2-3 |
| States of Matter | 2-3 |
| The d & f block Elements | 3-4 |
| Solutions | 2-3 |
Also Read:
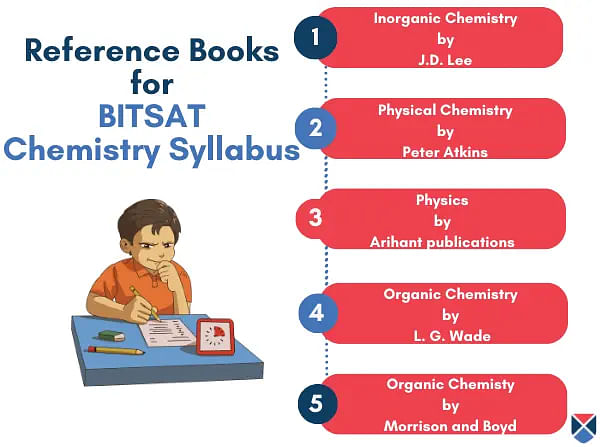
BITSAT Exam Chemistry Reference Books
Students are advised to include as many study materials as possible related to BITSAT Chemistry Syllabus 2025. This will boost the BITSAT preparation and provide a well-rounded perspective. Students must ensure that the BITSAT Books 2025 cover the foundational basis of Chemistry concepts, as well as in-depth themes.
The following books are a great source to study the BITSAT Chemistry 2025 syllabus:
- “Physical Chemistry” by Peter Atkins.
- “Inorganic Chemistry” by J. D. Lee.
- “Organic Cheimstry” by L. G. Wade Jr.
- “Chemistry” for Class 11 and 12 by NCERT.
- “15 Practice Papers” by Arihant Publications.
- “Physics” by Arihant Publications.
- “Organic Chemistry” by Morrison and Boyd.
BITSAT Chemistry 2025 Syllabus Preparation Tips
Different tips and tricks can be used to study the BITSAT Chemistry 2025 syllabus. Students must create a study plan considering some tips. If followed correctly, these tips can prepare students for the BITSAT exam properly:
- Combine the information from the BITSAT Chemistry Weightage 2025 and detailed syllabus to prioritise essential topics such as Basic Concepts, Equilibria and p-block elements.
- A study plan should also allot time to practicing reactions and equations since these are crucial elements of chemistry.
- Study the exam pattern to create a plan with proper time allocation to essential topics.
- Students must practice the BITSAT question papers from the previous year to identify crucial units. These units must be focused on for BITSAT preparation 2025. Last year’s papers will also provide a detailed look into the exam pattern and question format.
- Students must ensure the study plan is implemented a minimum of 5-6 months before. The last month of the study plan should involve revising topics and solving as many mock tests and question papers as possible.
- Using video tutorials from YouTube, Udemy, and Khan Academy to understand Chemistry concepts will boost students' understanding. These tutorials provide comprehensive explanations through visual aids to enhance preparation. Harshal-BITS Pilani on YouTube offers a thoroughly researched approach to studying essential topics.
- Students must include dedicated notebook for equations, terminologies and reactions into their study plan. Revising from this notebook helps students memorise core aspects of the Chemistry syllabus.
- In the study plan, students should allocate time to special topics such as atomic structures and chemical bonding.
- In case students only have 1-2 months to implement a study plan, they must focus on weightage wise topics first. They must also dedicate a minimum of 6-7 hours everyday to studying the BITSAT syllabus 2025.
- Ensure that there are no distractions while studying. Keep mobiles and laptops away while following the study plan.
- Different scientific materials such as textbooks, scientific journals, research papers, and articles can be used to study current happenings in the field.
- Solving the BITSAT mock tests will boost a student’s speed and precision during the exam.
- Study plans ensure time to balance study periods with resting periods. It will lead to a rejuvenated mind when students get back to studying. It will also provide a cool head during the exam, helping with stress management.
Quick Links:
| BITSAT Mock Test 2025 | How to Crack BITSAT 2025? |
| How to Prepare for BITSAT from Class 11? | BITSAT Important Topics 2025 |