Table of Contents
- NMC NEET 2025 Syllabus PDF
- NEET Syllabus Revised 2025
- NEET Syllabus 2025: Biology, Physics, Chemistry
- NEET Syllabus 2025 for Physics
- NEET Syllabus 2025 for Chemistry
- Syllabus for NEET 2025 for Biology
- Most Important Topics to Cover: Brief Analysis
- NEET 2025 Marking Scheme
- NEET 2025 Syllabus: Distribution of Questions from Syllabus
- NEET Syllabus 2025 Highlights
NEET Syllabus 2025 is released by the National Testing Agency (NTA) on the official website, along with an information bulletin. Last year, the NEET syllabus underwent significant adjustments. Thus, the NEET 2025 syllabus is available for candidates to prepare for the exam.
NEET syllabus 2025 PDF download is accessible before the NEET 2025 test, which is expected to be held on May 4, 2025. Aspirants studying for NEET 2025 can download the NEET syllabus 2025 PDF to stay up to date on the major chapters and topics for each subject of the NEET question paper, which are Physics, Chemistry, and Biology, respectively.
NEET revised syllabus PDFs for Physics, Chemistry, and Biology are officially released by the National Medical Commission. The Under Graduate Medical Education Board (UGMEB) of the National Medical Commission designed the syllabus. The syllabus has been revised with minor changes, after considering and reviewing the syllabus of state education boards such as COBSE NIOS, ICSE, CBSE and NCERT.
NMC NEET 2025 syllabus encompasses three subjects, i.e., Physics, Chemistry, and Biology (Botany & Zoology). The questions in the NEET Exam are based on the syllabus of Classes 11, and 12. Hence, it is best to use the NCERT textbooks from these classes to prepare for the NEET exam.
Also Read: NEET UG 2025 Syllabus (Released): Check Physics, Chemistry, Biology Topics
NMC NEET 2025 Syllabus PDF
As NEET is one of the most difficult competitive tests, applicants should be familiar with the NEET syllabus 2025 topic-wise. They should be aware of how many questions will be asked from each topic in the specific subject, as well as the NEET subject-wise weightage. NEET 2025 syllabus will include three subjects: Chemistry, Physics, and Biology.
NMC NEET 2025 syllabus PDF is released by the Indian regulatory body. Students preparing for the upcoming NEET exam 2025 are advised to go through the detailed NMC- revised syllabus for NEET through the link provided below.
| NEET Revised Syllabus PDF 2025 Download Link | Download Now |
Also Read: NEET Preparation Tips 2025
NTA NEET Syllabus 2025 PDF Download Subject-Wise
NEET syllabus 2025 PDF files containing detailed Physics, Chemistry and Biology topics of Class 11 and Class 12 are given below:
| Subject | Syllabus PDF |
|---|---|
| NEET Physics Syllabus | Download |
| NEET Chemistry Syllabus | Download |
| NEET Biology Syllabus | Download |
NEET Syllabus Revised 2025
Initially, the candidates had questions regarding modifications in the syllabus, and to clarify these doubts, NTA released an official notice, along with the revised NEET syllabus PDF download link. Candidates can download the official NMC NEET revised syllabus 2025 from the official website at nta.ac.in.
Candidates can check on the fresh NEET 2025 updates for detailed syllabus topics, new revisions, exam patterns and subject weightage in the sections below. The conducting authority has revised some of the topics from the syllabus for NEET 2025. Candidates must check the reduced syllabus topics shared below for different courses and classes.
Also Read: How to Complete 40% NEET UG Syllabus in 1 Month?
Reduced Topics in Physics & Chemistry
NMC has prescribed a new NEET syllabus 2025 topic-wise details. Some of the topics were removed and modified by NMC in Physics and Chemistry. The NEET syllabus reduced from each section is tabulated below.
| Topics Reduced from NEET Physics Syllabus | |
|---|---|
|
The motion of the System of Particles and Rigid Body |
The behaviour of Perfect Gas and Kinetic Theory |
| Electromagnetic Waves | |
| Topics Reduced from NEET Chemistry Syllabus | |
| S - Block Element (Alkali and Alkaline earth metals) | Some p - Block Elements |
| Environmental Chemistry | Polymers |
| Aldehydes, Ketones and Carboxylic Acids | Haloalkanes and Haloarenes |
| General Principles and Processes of Isolation of Elements | Chemistry in everyday life |
| Alcohols, Phenols and Ethers | Electrochemistry |
| Solid State | Surface Chemistry |
Also Read: How to Prepare Physics for NEET 2025?
Topics Reduced from Class 12 Syllabus 2025
Keeping in mind the loss of studies due to the outbreak of COVID-19, CBSE decided to reduce several important topics from the syllabus of classes 11 and 12. Hence, the NEET syllabus was reduced by NTA in the revised version. This move aims to lift the burden of covering an expansive NEET syllabus 2025 off students' shoulders in such a situation. The topics reduced from the syllabus of Biology are tabulated below:
| Topics Reduced from NEET Biology Syllabus | |
|---|---|
| Human Physiology | Ecosystems |
| Plant Physiology | Environmental Issues |
| Evolution | Reflex Action, Sensory Perception, Sense Organs |
| Transportation in Plants, Digestion, and Absorption | Elementary Structure and Function of Eye and Ear |
| Locomotion and Movement | |
NEET Reduced Syllabus Chemistry 2025
Some topics removed from the NEET Chemistry syllabus are activation energy, carbon and its compounds, and the Arrhenius equation. For a more comprehensive list, aspirants may view the topics reduced from the Syllabus of Chemistry tabulated below:
| Topics Omitted from Chemistry Syllabus of Class 11 and 12 | |
|---|---|
| Arrhenius Equation | Basic Metallurgical Processes; Corrosion and Prevention |
| Activation Energy | Carbon and its Compounds |
| Basic Atomic Structure | Nomenclature of Carbon and its Compounds |
| Chemical Substances - Metals and Non -Metals | Difference between saturated and unsaturated hydrocarbons |
NEET Reduced Syllabus Physics 2025
Physics is the most difficult subject among the NEET subjects. Some topics that were removed from the NEET Physics syllabus 2025 are the Doppler effect, effects of the current, electric generators, etc. A full list of the topics omitted from the Physics Syllabus for grades 11 and 12 have been discussed below:
| Physics Topics Reduced from Classes 11 and 12 Syllabus | |
|---|---|
| Doppler Effect | Effects of Current |
| Inertia and Momentum | Magnetic Effects of Electric Current |
| Newton's Laws of Motion | Electric Generator |
| Kepler's Law of Planetary Motion | Domestic Electric Circuits |
| Direct Current, Alternating Current: Frequency of AC and Advantage of AC over DC | |
It has not been confirmed whether questions from any of these topics will be asked in the NEET exam 2025 or not. However, candidates must note that the NEET syllabus was reduced by the NTA in the revised version. Any information regarding the NEET 2025 update of the syllabus will be provided on this page.
Also Read: NEET Syllabus 2025: Download Subject-wise PDF
NEET Syllabus 2025: Biology, Physics, Chemistry
Candidates are required to cover three subjects under the NMC NEET syllabus 2025. The curriculum for NEET consists of the following subjects: Physics, Chemistry, Zoology & Botany. Some important facts to keep in mind for the NEET 2025 syllabus PDF by NTA have been mentioned below.
- NMC prescribed the NTA NEET subjects 2025, based on the curriculum prepared by the Under Graduate Medical Education Board (UGMEB).
- Previously, the NTA NEET exam syllabus 2025 was prepared with the collaborative work of the Central Board of Secondary Education (CBSE), and the National Council of Education Research and Training (NCERT).
- Apart from CBSE's curriculum, the syllabus of NEET 2025 will be finalized after reviewing the syllabus from several different state boards.
The subject-wise NEET updated syllabus 2025 is available for the candidate's reference for Physics, Chemistry and Biology. Applicants are advised to assess the topics for all NEET subjects. In addition, candidates must go through the updated NMC NEET 2025 syllabus for any fresh modifications before starting their exam preparation to allocate time to important topics and ace the exam. Candidates must try to gain access to NMC NEET syllabus books for a thorough preparation process as well.
NEET Syllabus 2025 for Physics
The aspirants must be acquainted with the fact that the NEET syllabus 2025 by NMC for each subject is quite vast. The question paper for NEET 2025 shall contain questions from Physics from the curriculum of classes 11 and 12. Some of the units covered under the NEET reduced syllabus for Physics are given below:
- Physics and Measurement
- Kinematics
- Laws of Motion
- Work, Energy, and Power
- Rotational Motion
- Gravitation
- Properties of Bulk Matter/Solid and Liquid
- Heat and Thermodynamics
- Kinetic Theory and Behaviour of Perfect Gases
- Oscillations and Waves
- Electrostatics
- Current Electricity
- Magnetic Effects of Current and Magnetism
- Electromagnetic Induction and Alternating Currents
- Electro Magnetic Waves
- Optics
- Dual Nature of Matter and Radiation
- Atoms and Nuclei
- Electronic Devices
- Experimental Skills
Also Read: How To Score 720 In NEET? Preparation Tips, Strategies
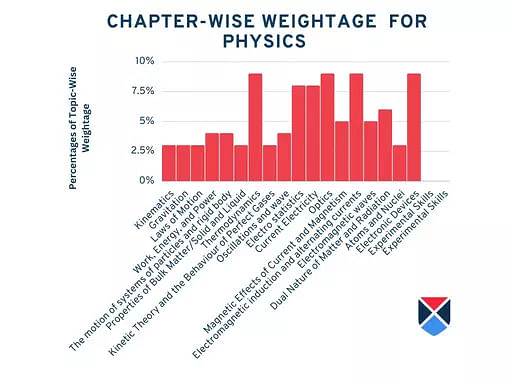
NEET Syllabus 2025 Tentative Chapter-Wise Weightage Physics
The tentative chapter-wise weightage for NEET 2025 PDF by NTA is tabulated below:
| Chapters | Weightage |
| Physical-world and measurement | 2% |
| Kinematics | 3% |
| Gravitation | 3% |
| Laws of Motion | 3% |
| Work, Energy, and Power | 4% |
| The motion of systems of particles and rigid body | 4% |
| Properties of Bulk Matter/Solid and Liquid | 3% |
| Thermodynamics | 9% |
| Kinetic Theory and the Behaviour of Perfect Gases | 3% |
| Oscillations and wave | 4% |
| Electro statistics | 8% |
| Current Electricity | 8% |
| Optics | 9% |
| Magnetic Effects of Current and Magnetism | 5% |
| Electromagnetic induction and alternating currents | 9% |
| Electromagnetic waves | 5% |
| Dual Nature of Matter and Radiation | 6% |
| Atoms and Nuclei | 3% |
| Electronic Devices | 9% |
| Experimental Skills | - |
Best Books for NEET 2025 Physics
The best books recommended for the preparation of the Physics syllabus of NEET 2025 are tabulated below. Candidates must go through these books and other relevant materials that cover the important topics to study the NEET Physics Syllabus PDF 2025.
| Book Title | Author/Publisher |
| Concepts of Physics Parts 1 & 2 | H C Verma |
| Fundamentals of Physics | Halliday, Resnick and Walker |
| Objective Physics for Medical Entrance (Vols 1 & 2) | D C Pandey |
| Objective NCERT at your Fingertips | MTG |
| Physics MCQ | D Mukherjee |
| Problems in General Physics | I E Irodov |
| Class 11 & 12 Textbooks | NCERT |
NEET Syllabus 2025 for Chemistry
Candidates can check the detailed NMC NEET subjects list for Chemistry. Given below is the NEET 2025 reduced syllabus for Chemistry. The PDF NEET syllabus for Chemistry contains topics from the syllabus of classes 11 and 12. The topics covered in the Chemistry NEET exam syllabus 2025 are listed below:
Physical Chemistry
- Some Basic Concepts of Chemistry
- Atomic Structures
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solutions
- Equilibrium
- Redox Reactions & Electro Chemistry
- Chemical Kinetics
Inorganic Chemistry
- Classification of Elements and Periodicity in Properties
- P - Block Elements
- d and f Block Elements
- Coordination Compounds
Ogrganic Chemistry
- Purification & Characterisation of Organic Compounds
- Some Basic Principles and Techniques of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Biomolecules
- Principles Related to Practical Chemistry
Also Read: Updated NEET Chemistry Syllabus, Newly Added and Deleted Topics
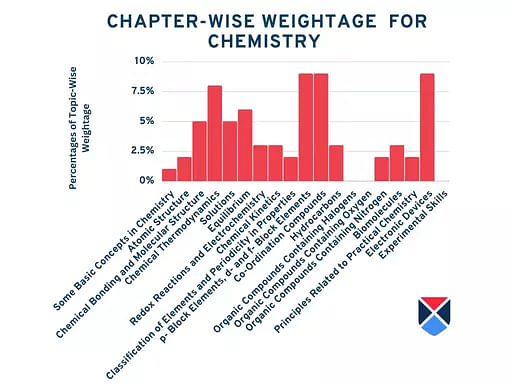
NEET 2025 Chemistry Syllabus Tentative Chapter-Wise Weightage
The weightage of each unit in the NEET 2025 syllabus for Chemistry will be announced soon for the candidate's reference:
| Chapters | Weightage |
| Some Basic Concepts in Chemistry | 1% |
| Atomic Structure | 2% |
| Chemical Bonding and Molecular Structure | 5% |
| Chemical Thermodynamics | 8% |
| Solutions | 5% |
| Equilibrium | 6% |
| Redox Reactions and Electrochemistry | 3% |
| Chemical Kinetics | 3% |
|
Classification of Elements and Periodicity in Properties
|
2% |
| p- Block Elements, d- and f- Block Elements | 9% |
| Co-Ordination Compounds | 9% |
| Hydrocarbons | 3% |
| Organic Compounds Containing Halogens | - |
| Organic Compounds Containing Oxygen | - |
| Organic Compounds Containing Nitrogen | 2% |
| Biomolecules | 3% |
| Principles Related to Practical Chemistry | 2% |
Also Read: How to Prepare Chemistry Section for NEET 2025?
Best Books for NEET 2025 Chemistry
The best books recommended for the preparation of Chemistry subject for NEET reduced syllabus 2025 are tabulated below. Candidates must go through these books that cover all the fundamental topics to prepare for the NEET 2025 Chemistry exam.
| Book Title | Author/Publisher |
| 40 Days Chemistry for NEET | Sudhanshu Thakur |
| Boyd for Organic Chemistry | Robert Thornton Morrison / Rebert Neilson Boyd |
| Concise Inorganic Chemistry | J D Lee |
| Objective NCERT at Your Fingertips | MTG |
| Organic Chemistry | Himanshu Pandey |
| Class 11 & 12 Textbooks | NCERT |
Syllabus for NEET 2025 for Biology
Given below is the NEET Biology syllabus 2025. The candidates must note that the NEET biology syllabus PDF for 2025 also contains topics from classes 11 and 12. The topics covered in the NEET biology syllabus 2025 are listed below:
- Diversity in the Living World
- Structural Organisation in Animals and Plants
- Cell Structure and Function
- Plant Physiology
- Human physiology
- Reproduction
- Genetics and Evolution
- Biology and Human Welfare
- Biotechnology and Its Applications
- Ecology and Environment
Also Read: Best Day Wise Revision Strategy for NEET 2025
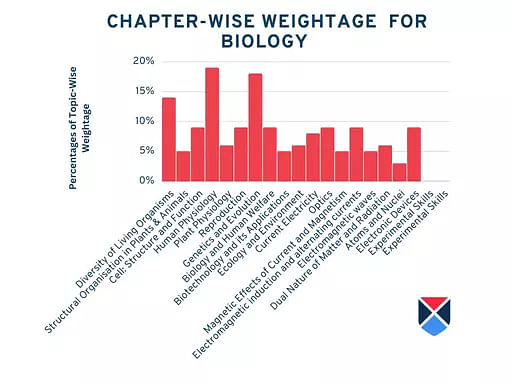
NEET 2025 Biology Syllabus Tentative Chapter-Wise Weightage
The approximate weightage given to each chapter under NEET biology syllabus is mentioned below:
| Chapters | Weightage |
| Diversity of Living Organisms | 14% |
| Structural Organisation in Plants & Animals | 5% |
| Cell: Structure and Function | 9% |
| Human Physiology | 19% |
| Plant Physiology | 6% |
| Reproduction | 9% |
| Genetics and Evolution | 18% |
| Biology and Human Welfare | 9% |
| Biotechnology and its Applications | 5% |
| Ecology and Environment | 6% |
Best Books for NEET 2025 Biology
The best books recommended for the preparation of NEET Biology Syllabus have been highlighted below. Candidates must go through these books that cover all the important topics for the exam.
| Book Title | Author/Publisher |
|---|---|
| 40 Days Biology for NEET | S Chakravarty |
| Objective Botany | Ansari |
| Exploring Biology (Vol 1 & 2) | Arihant Publications |
| Objective NCERT at your Fingertips | MTG |
| Pradeep's Publications Biology | Pradeep's Publications |
| True Man's Objectives Biology for NEET | M P Tyagi |
| Class 11 & 12 Textbooks | NCERT |
Also Read: Important Diagrams For Class 11 Botany for NEET Biology
Most Important Topics to Cover: Brief Analysis
Each NEET exam subject has a vast syllabus, and it is not possible to touch on every topic. In the tables below, we have compiled an analysis based on past year trends that will help candidates to prepare better for the exams and cover the NEET 2025 syllabus revised by NMC for the academic year 2025.
Detailed Analysis of NEET Physics
Out of the several topics in the NEET Physics syllabus, the most trending ones have been tabulated to understand and better prepare for the exam. From analyzing the trends, it is visible that Mechanics had a higher weightage in NEET Exam as compared to the other topics.
| Topics | 2019 | 2018 | 2017 |
| Mechanics | 40% | 31% | 36% |
| Magnetism | 16% | 9% | 13% |
| Electricity | 13% | 13% | 9% |
| Modern Physics | 11% | 18% | 16% |
| Heat and Thermodynamics | 9% | 11% | 11% |
| Optics | 9% | 11% | 11% |
| Waves | 2% | 7% | 4% |
Detailed Analysis of NEET Chemistry
Out of all the NEET subjects, Chemistry has a vast syllabus, including Physical, Inorganic and Organic chemistry. Out of these multiple topics in the NEET Chemistry syllabus, the most trending ones have been tabulated to understand which areas need more focus to prepare for the exam.
| Topics | 2019 | 2018 | 2017 |
| Physical Chemistry (XI) | 24% | 24% | 22% |
| Inorganic Chemistry (XI) | 18% | 16% | 4% |
| Physical Chemistry (XII) | 18% | 11% | 16% |
| Organic Chemistry (XII) | 16% | 20% | 20% |
| Inorganic Chemistry (XII) | 13% | 16% | 22% |
| Organic Chemistry (XI) | 11% | 13% | 16% |
From analyzing the trends, it is visible that the Physical Chemistry from class XI has had domination compared to the others.
Detailed Analysis of NEET Biology: Botany
Among the NEET subjects, Biology is the highest-scoring section of the exam. Being a medical entrance exam, a lot of hard work is put into the Botany section of the NEET UG syllabus 2025. The previous year's trends are tabulated below.
| Topics | 2019 | 2018 | 2017 |
| Ecology | 19% | 17% | 17% |
| Genetics | 17% | 15% | 23% |
| Plant Physiology | 17% | 11% | 11% |
| Structure & Func. of Cell | 15% | 17% | 8% |
| Biology in Human Welfare | 8% | 2% | 6% |
| Diversity of Life | 8% | 17% | 13% |
| Reproduction & Sexual Reproduction | 8% | 10% | 9% |
| Structural Organization of Plants | 8% | 11% | 13% |
Also Read: 6 Month Most Realistic Strategy For NEET 2025 Exam
Detailed Analysis of NEET Biology: Zoology
Being a medical entrance exam, the Zoology section of the NEET Exam is essential. The previous year's trends have been tabulated below for the candidate's reference.
| Topics | 2019 | 2018 | 2017 |
| Human Physiology | 33% | 38% | 41% |
| Animal Husbandry & Biotechnology | 17% | 14% | 18% |
| Human Reproduction & Reproductive Health | 14% | 16% | 14% |
| Biomolecules | 10% | 3% | 5% |
| Evolution: Theories & Evidence | 9% | 8% | 3% |
| Human Health & Disease | 7% | 8% | 5% |
| Animal Kingdom | 5% | 8% | 11% |
| Structural Organisation in Animals | 5% | 5% | 5% |
NEET 2025 Marking Scheme
NEET 2025 marking scheme is mentioned below.
- Each question is of 4 marks.
- Candidates answering the question correctly will be awarded 4 marks against each question.
- For every wrong answer, 1 mark will be deducted.
- Unattempted questions will have no negative marking.
Also Read: Must-Have Books to Score 700+ in NEET UG 2025
NEET 2025 Syllabus: Distribution of Questions from Syllabus
NEET 2025 distribution of marks based on subjects for class XI and class XII are mentioned below.
NEET 2025 Syllabus: Class XI and XII
| NEET 2025 Syllabus Class 11 | ||
| Subjects | Number of Questions | Total Marks |
| Physics | 22 | 88 |
| Chemistry | 23 | 92 |
| Biology | 46 | 184 |
| Total | 91 | 364 |
| NEET 2025 Syllabus Class 12 | ||
| Physics | 23 | 92 |
| Chemistry | 22 | 88 |
| Biology | 44 | 176 |
| Total | 89 | 356 |
| NEET 2025 Syllabus Total Marks | ||
| Physics | 45 | 180 |
| Chemistry | 45 | 180 |
| Biology | 90 | 360 |
| Total | 180 | 720 |
NEET Syllabus 2025 Highlights
Having a proper knowledge of the syllabus of NEET 2025 is of utmost importance. NEET UG 2025 syllabus includes subjects from classes 11th, and 12th. Knowledge about NEET 2025 important chapters is essential for an aspirant preparing for the upcoming exam to crack it.
In addition, an important note that candidates must remember is that the NEET 2025 syllabus has been revised by NMC for 2025. Candidates can find the revised and detailed NEET 2025 syllabus PDF in the article below.
- Subjects- NEET Syllabus for 2025 shall comprise topics from Physics, Chemistry and Biology (Zoology+Botany).
- Sections- The NEET 2025 exam pattern will have four sections viz. Physics, Chemistry, Zoology & Botany.
- Difficulty Level- The questions in NEET 2025 will be based on Class XI and Class XII (Physics, Chemistry & Biology) subjects.
- No. of Questions- The question paper will have 45 objective-type questions from every section, viz. Physics, Chemistry, and Biology. The total number of questions would hence be 180.
- Marks- NEET 2025 question paper would be 720 marks.
- Marking Scheme- Every right answer will fetch the candidate 4 marks, while 1 mark will be deducted for every wrong answer.
FAQs on NEET Syllabus
Q: What does the NEET 2025 syllabus include?
The subject-wise topics for the subjects Physics, Chemistry and Biology have included in NEET syllabus 2025.
Q: How many questions are asked in the NEET 2025 Chemistry section?
Based on the exam pattern set by NMC, 50 questions will be asked in the NEET 2025 Chemistry section, out of which the candidates have to attempt 45 questions for a total of 180 marks.
Q: Where can I get the NEET syllabus 2025 PDF in Hindi?
The official NEET syllabus 2025 released by NMC is in English. Candidates who opted for Hindi or other regional languages as their medium for the exam could compare the class 11+12 syllabus with the NEET syllabus and approach their teachers/mentors for the syllabus PDF in Hindi or other required languages.
Q: What is the syllabus of NEET 2025?
The syllabus of NEET 2025 is comprehensive and covers topics from the subjects of Biology, Physics, and Chemistry from classes 11 and 12.
Q: What syllabus comes in NEET 2025?
The examination structure for NEET 2025 incorporates a well-rounded distribution of marks, covering Physics, Chemistry, and Biology.
Q: Is the NEET syllabus changed?
NEET syllabus has undergone changes in Physics, Chemistry, and Biology. Some chapters were removed, while others were added or modified. The National Medical Commission (NMC) released the updated syllabus in response to a plea filed in the Delhi High Court.