The council designs the ICSE class 10 chemistry syllabus, which contains complete details on the course structure and sub-topics related to the Chemistry subjects. This updated syllabus will help the students learn the concepts for class 11 and also for the engineering and medical entrance exams.
Furthermore, the ICSE class 10 chemistry syllabus 2023-24 has 9 chapters. The total mark for the theory paper is 80, and for internal assessment, it is 20 marks. Students will be allotted two hours to write the paper.
Table of Contents
ICSE Class 10 Chemistry Syllabus 2023-24: PDF Download
The updated ICSE syllabus will give an overview of the exact question paper pattern and the marks distribution. The PDF link for the ICSE class 10 Chemistry syllabus is provided below, which will help the students understand the essential topics that must be prepared effectively.
Read more: ICSE Class 10 Chemistry Reduced Syllabus
ICSE Class 10 Chemistry 2023-24 Syllabus: Chapter-Wise Topics
The ICSE class 10 Chemistry syllabus covers fundamental concepts, including periodic properties, chemical bonding, acids, bases, salts, analytical chemistry, mole concept, stoichiometry, electrolysis, metallurgy, and organic chemistry. The detailed chapter-wise Chemistry syllabus class 10 ICSE 2024 is below for the student's reference.
ICSE Class 10 Chemistry Syllabus for Chapter 1: Periodic Properties and variations of Properties – Physical and Chemical
Refer to topics mentioned below which this chapter covers.
ICSE Class 10 Chemistry Chapter 1: Periodic Properties and variations of Properties
| Topics |
Sub-Topics |
Periodic properties and their variations in
groups and periods. |
Definitions and trends of the following periodic properties in groups and periods
should be studied:
- atomic size
- metallic character
- non-metallic character
- ionisation potential
- electron affinity
- electronegativity
|
Periodicity on the basis of atomic number for
elements. |
- The study of modern periodic table up to period 3 (students to be exposed to the complete modern periodic table but no questions will be asked on elements beyond period 3 – Argon);
- Periodicity and other related properties to be explained on the basis of nuclear charge and shells (not orbitals).
|
Chemistry Syllabus Class 10 ICSE 2024 2023-24 for Chapter 2: Chemical Bonding
The detailed topics and sub-topics mentioned in the ICSE class 10 Chemistry syllabus chapter 2 is tabulated below for the students reference.
ICSE Class 10 Chemistry Chapter 2: Chemical Bonding
| Topics |
Sub-Topics |
| Electrovalent bonding |
- Electron dot structure of Electrovalent compounds NaCl, MgCl2, CaO.
- Characteristic properties of electrovalent compounds – state of existence, melting and boiling points, conductivity (heat and electricity), dissociation in solution and in molten state to be linked with electrolysis.
|
| Covalent Bonding |
- Electron dot structure of covalent molecules on the basis of duplet and octet of electrons (example: hydrogen, chlorine, nitrogen, ammonia, carbontetrachloride, methane.
- Polar Covalent compounds – based on difference in electronegativity: Examples – HCl and H2O including structures.
- Characteristic properties of Covalent compounds – state of existence, melting and boiling points, conductivity (heat and electricity), ionisation in solution.
|
| Coordinate Bonding |
- Definition
- The lone pair effect of the oxygen atom of the water molecule and the nitrogen atomof the ammonia molecule to explain the
formation of H3O+ and OH- ions in water and NH4+ion
|
ICSE Class 10 Chemistry Syllabus for Chapter 3: Study of Acids, Bases and Salts
Tabulated below are the topics and sub-topics ICSE class 10 Chemistry syllabus chapter 3 covers which students must refer to.
ICSE Class 10 Chemistry Chapter 3: Study of Acids, Bases and Salts
| Topics |
Sub-Topics |
| Definations |
- Simple definitions in terms of the molecules and their characteristic properties
|
Ions present in mineral acids, alkalis and salts
and their solutions; use of litmus and pH paper
to test for acidity and alkalinity. |
- Examples with equation for the ionisation/dissociation of ions of acids, bases and salts.
- Acids form hydronium ions (only positive ions) which turn blue litmus red, alkalis form hydroxyl ions (only negative ions) with water which turns red litmus blue. Salts are formed by partial or complete replacement of the hydrogen ion of an acid by a metal. (To be explained with suitable examples).
- Introduction to pH scale to test for acidity, neutrality and alkalinity by using pH paper or Universal indicator.
|
| Definition of salt; types of salts. |
- Types of salts: normal salts, acid salt, basic salt, definition and examples.
|
| Action of dilute acids on salts. |
- Decomposition of hydrogen carbonates, carbonates, sulphites and sulphides by appropriate acids with heating if necessary. (Relevant laboratory work must be done).
|
Methods of preparation of Normal salts with
relevant equations. |
Methods included are:
- Direct combination
- Displacement
- Precipitation (double decomposition)
- Neutralization of insoluble base
- Neutralisation of an alkali (titration)
- Action of dilute
|
ICSE Class 10 Chemistry Syllabus 2023-24 for Chapter 4: Analytical Chemistry
Refer to the table mentioned below to understand the ICSE class 10 Chemistry syllabus chapter 4.
ICSE Class 10 Chemistry Chapter 4: Analytical Chemistry
| Topics |
Sub-Topics |
| Action of Ammonium Hydroxide and Sodium Hydroxide on solution of salts |
- Colour of salt and its solution; formation and colour of hydroxide precipitated for solutions of salts of Ca, Fe, Cu, Zn and Pb;
special action of ammonium hydroxide on solutions of copper salt and sodiumhydroxide on ammonium salts.
- On solution of salts: Colour of salt and its solution, Action on addition of SodiumHydroxide to solution of Ca, Fe, Cu, Zn, and Pb salts drop by drop in excess. Formation and colour of hydroxide precipitated to be highlighted with the help of equations, Action on addition of AmmoniumHydroxide to solution of Ca, Fe, Cu, Zn, and Pb salts drop by drop in excess. Formation and colour of hydroxide precipitated to be highlighted with the help of equations.
- Special action of AmmoniumHydroxide on solutions of copper salts and sodium hydroxide on ammonium salts.
|
| Action of alkalis (NaOH, KOH) on certain metals, their oxides and hydroxides |
- The metals must include aluminium, zinc and lead, their oxides and hydroxides, which react with caustic alkalis (NaOH, KOH), showing the amphoteric nature of these substances
|
Chemistry Syllabus Class 10 ICSE 2024 for Chapter 5: Mole Concept and Stoichiometry
Students have to study the below topics in this chapter from the ICSE class 10 Chemistry syllabus:
ICSE Class 10 Chemistry Chapter 5: Mole Concept and Stoichiometry
| Topics |
Sub-Topics |
| Gay Lussac’s Law of Combining Volumes; Avogadro’s Law. |
- Idea of mole – a number just as a dozen, a gross (Avogadro’s number).
- Avogadro’s Law - statement and explanation.
- Gay Lussac’s Law of Combining Volumes. – Statement and explanation.
- Understanding molar volume- “the mass of 22.4 litres of any gas at S.T.P. is equal to its molar mass”. (Questions will not be set on formal proof but may be taught for clear understanding). Simple calculations based on the molar volume and Gay Lussac’s law.
|
| Refer to the atomicity of hydrogen, oxygen, nitrogen and chlorine (proof not required). |
- The explanation can be given using equations for the formation of HCl, NH3, and NO.
|
| Vapour Density and its relation to relative molecular mass: |
- Molecular mass = 2 vapour density (formal proof not required)
- Deduction of simple (empirical) and molecular formula from: The percentage composition of a compound, The masses of combining elements.
|
| Mole and its relation to mass. |
- Relating mole and atomic mass; arriving at gram atomic mass and then gram atom; atomic mass is a number dealing with one atom; gram atomic mass is the mass of one mole of atoms.
- Relating mole and molecular mass arriving at gram molecular mass and gram molecule – molecular mass is a number dealing with a molecule, gram molecular mass is the mass of one mole of molecules.
- Simple calculations based on relation of mole to mass, volume and Avogadro’s number.
|
| Simple calculations based on chemical equations |
- Related to weight and/or volumes of both reactants and products
|
ICSE Class 10 Chemistry Syllabus 2023-24 for Chapter 6: Electrolysis
Refer to the table mentioned below to understand the chapter 6 ICSE class 10 chemistry syllabus 2024.
ICSE Class 10 Chemistry Chapter 6: Electrolysis
| Topics |
Sub-Topics |
| Electrolytes and non-electrolytes |
Definitions and examples |
| Substances containing molecules only, ions only, both molecules and ions |
- Substances containing molecules only ions only, both molecules and ions.
- Examples; relating their composition with their behaviour as strong and weak electrolytes as well as non-electrolytes.
|
| Defination |
- Definition and explanation of electrolysis, electrolyte, electrode, anode, cathode, anion, cation, oxidation and reduction (on the basis of loss and gain of electrons).
|
An elementary study of the migration of ions, with reference to the factors influencing selective discharge of ions (reference should be made to the activity series as indicating the
tendency of metals, e.g. Na, Mg, Fe, Cu, to form ions) illustrated by the electrolysis of |
- Molten lead bromide
- acidified water with platinum electrodes
- Aqueous copper (II) sulphate with copper electrodes; electron transfer at the electrodes.
|
| Applications of electrolysis |
- Electroplating with nickel and silver, choice of electrolyte for electroplating.
- Electro refining of copper.
|
ICSE Class 10 Chemistry Syllabus for Chapter 7: Metallurgy
The ICSE class 10 2024 Chemistry syllabus is tabulated below for chapter 7.
ICSE Class 10 Chemistry Chapter 7: Metallurgy
| Topics |
Sub-Topics |
| Occurrence of metals in nature |
- Mineral and ore - Meaning only.
- Common ores of iron, aluminium and zinc.
|
| Stages involved in the extraction of metals |
- Dressing of the ore – hydrolytic method, magnetic separation, froth flotation method.
- Conversion of concentrated ore to its oxide- roasting and calcination (definition, examples with equations).
- Reduction of metallic oxides- some can be reduced by hydrogen, carbon and carbon 114 monoxide (e.g. copper oxide, lead (II) oxide, iron (III) oxide and zinc oxide) and some cannot (e.g. Al2O3, MgO) - refer to activity series). Active metals by electrolysis e.g. sodium, potassium and calcium. (reference only). Equations with conditions should be given.
- Electro refining – reference only
|
| Extraction of Aluminium. |
- Chemical method for purifying bauxite by using NaOH – Baeyer’s Process.
- Electrolytic extraction – Hall Heroult’s process: Structure of electrolytic cell - the various components as part of the electrolyte, electrodes and electrode reactions.
|
| Alloys – composition and uses. |
- Stainless steel, duralumin, brass, bronze, fuse metal / solder
|
ICSE Class 10 Chemistry Syllabus 2023-24 for Chapter 8: Study of Compounds
The chapter 8 ICSE class 10 Chemistry syllabus for 2024 is mentioned below for student's reference.
ICSE Class 10 Chemistry Chapter 8: Study of Compounds
| Topics |
Sub-Topics |
| Hydrogen Chloride |
Hydrogen chloride: preparation of hydrogen chloride from sodium chloride; refer to the density and solubility of hydrogen chloride (fountain experiment); reaction with ammonia; acidic properties of its solution.
- Preparation of hydrogen chloride from sodium chloride; the laboratory method of preparation can be learnt in terms of reactants, product, condition, equation, diagram or setting of the apparatus, procedure, observation, precaution, collection of the gas and identification.
- Simple experiment to show the density of the gas (Hydrogen Chloride) –heavier than air.
- Solubility of hydrogen chloride (fountain experiment); setting of the apparatus, procedure, observation, inference.
- Method of preparation of hydrochloric acid by dissolving the gas in water- the
special arrangement and the mechanismby which the back suction is avoided should be learnt.
- Reaction with ammonia
- Acidic properties of its solution - reaction with metals, their oxides, hydroxides and carbonates to give their chlorides; decomposition of carbonates, hydrogen carbonates, sulphides, sulphites.
- Precipitation reactions with silver nitrate solution and lead nitrate solution.
|
| Ammonia |
Ammonia: its laboratory preparation fromammonium chloride and collection; ammonia
from nitrides like Mg3N2 and AlN and ammonium salts. Manufacture by Haber’s Process; density and solubility of ammonia (fountain experiment); aqueous solution of ammonia; its reactions with hydrogen chloride and with hot copper (II) oxide and chlorine; the burning of ammonia in oxygen; uses of ammonia.
- Laboratory preparation from ammoniumchloride and collection; (the preparation to be studied in terms of, setting of the apparatus and diagram, procedure, observation, collection and identification)
- Ammonia from nitrides like Mg3N2 and AlN using warm water. Ammonia from ammonium salts using alkalies.
|
| Nitric Acid |
Nitric Acid: one laboratory method of preparation of nitric acid from potassium nitrate or sodium nitrate. Large scale preparation. Nitric acid as an oxidizing agent.
- Laboratory preparation of nitric acid from potassium nitrate or sodium nitrate; the laboratory method to be studied in terms of reactants, products, conditions, equations, setting up of apparatus, diagram, precautions, collection and identification.
- Manufacture of Nitric acid by Ostwald’s process (Only equations with conditions where applicable).
- As an oxidising agent: its reaction with copper, carbon, sulphur
|
| Sulphuric Acid |
Large scale preparation, its behaviour as an acid when dilute, as an oxidizing agent when concentrated - oxidation of carbon and sulphur; as a dehydrating agent - dehydration of sugar and copper (II) sulphate crystals; its non-volatile nature.
- Manufacture by Contact Process Equations with conditions where applicable).
- Its behaviour as an acid when dilute - reaction with metal, metal oxide, metal hydroxide, metal carbonate, metal bicarbonate, metal sulphite, metal sulphide.
- Concentrated sulphuric acid as an oxidizing agent - the oxidation of carbon and sulphur.
- Concentrated sulphuric acid as a dehydrating agent- (a) the dehydration of
sugar (b) Copper (II) sulphate crystals.
- Non-volatile nature of sulphuric acid - reaction with sodium or potassiumchloride and sodium or potassium nitrate
|
ICSE Class 10 Chemistry Syllabus for Chapter 9: Organic Chemistry
Students have to study the below topics in this chapter from the ICSE class 10 Chemistry syllabus:
ICSE Class 10 Chemistry Chapter 9: Organic Chemistry
| Topics |
Sub-Topics |
| Introduction to Organic compounds |
- Unique nature of Carbon atom – tetra valency, catenation.
- Formation of single, double and triple bonds, straight chain, branched chain, cyclic compounds (only benzene).
|
| Structure and Isomerism |
- Structure of compounds with single, double and triple bonds.
- Structural formulae of hydrocarbons. Structural formula must be given for: alkanes, alkenes, alkynes up to 5 carbon atoms.
- Isomerism – structural (chain, position)
|
| Homologous series characteristics with examples |
- Alkane, alkene, alkyne series and their gradation in properties and the relationship with the molecular mass or molecular formula
|
| Simple nomenclature |
- Simple nomenclature of the hydrocarbons with simple functional groups – (double bond, triple bond, alcoholic, aldehydic, carboxylic group) longest chain rule and smallest number for functional groups rule – trivial and IUPAC names (compounds with only one functional group).
|
| Hydrocarbons: alkanes, alkenes, alkynes |
- Alkanes - general formula; methane (greenhouse gas) and ethane - methods of preparation from sodium ethanoate (sodium acetate), sodium propanoate 116 (sodium propionate), from iodomethane (methyl iodide) and bromoethane (ethyl bromide). Complete combustion of
methane and ethane, reaction of methane and ethane with chlorine through substitution.
- Alkenes – (unsaturated hydrocarbons with a double bond); ethene as an example. Methods of preparation of ethene by dehydro halogenation reaction and dehydration reactions.
- Alkynes - (unsaturated hydrocarbons with a triple bond); ethyne as an example of alkyne; Methods of preparation from calcium carbide and 1,2 dibromoethane ethylene dibromide).
|
Chemistry Syllabus Class 10 ICSE 2024: Marking Scheme
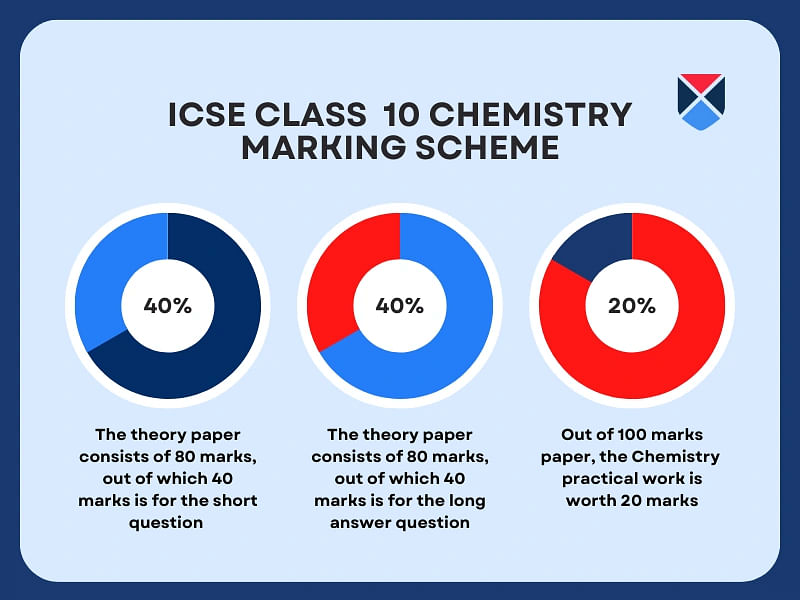
The Chemistry syllabus class 10 ICSE 2024 is distributed into the theory and internal assessment work. The theory paper will be divided into sections: Section I and Section II. Provided below are the detailed question paper pattern and marking scheme for ICSE class 10 Chemistry:
ICSE Class 10 Chemistry Marking Scheme
| Marks |
Details |
Number of Questions |
| 80 marks |
Section 1: Short questions |
Multiple |
| Section 2: Long answer questions |
6 Questions |
| 20 Marks |
Practical Assessment |
- |
Refer to the image below to understand the marking scheme of the ICSE class 10 Chemistry syllabus follows.
Read More: ICSE Class 10 Passing Marks
ICSE Class 10 Chemistry Syllabus 2023-24: Internal Assessment
The practical exams for the ICSE class 10 carry 20 marks. School authorities are responsible for conducting the internal assessment exams. Some of the points are mentioned below:
- The practical assessment will include recognising and identifying certain gases and ions, such as hydrogen, oxygen, carbon dioxide, chlorine, ammonia, and various ions, such as calcium, copper, iron, lead, zinc, etc.
- Candidates are also expected to do experiments such as making solutions of unknown substances, testing for acidity and basicity of given solutions, and reacting substances with concentrated hydrochloric acid to make observations and deductions.
Read More: ICSE Class 10 Previous Year Question Papers
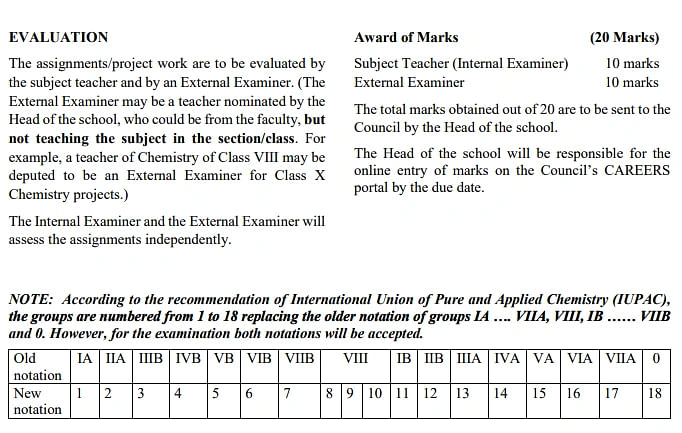
ICSE Class 10 Chemistry Syllabus: Evaluation
The subject teacher and an External Examiner evaluate the Chemistry syllabus class 10 ICSE 2024 assignments. Some of the essential marks students must understand are referred to in the table mentioned below:
ICSE Class 10 Chemistry Marks Evaluation
| Evaluation Component |
Internal Examiner Marks |
External Examiner Marks |
Total Marks |
| Assignments/ Project Work Evaluation |
10 |
10 |
20 |
Refer to the image mentioned below to understand the ICSE class 10 Chemistry syllabus marks evaluation.











![Motilal Nehru National Institute of Technology, [MNNIT] Allahabad](https://media.getmyuni.com/azure/college-image/small/motilal-nehru-national-institute-of-technology-mnnit-allahabad.webp)

















